Surprising Zinc !
Rob Putnam , ILZRO and Michael Martin , IZA
With many thousands of patents and literally
millions of products to its credit , zinc played a
supporting role in many of the major inventions
of the 20th Century
Zinc in Space
As a protective coating for steel, zinc has no equal. The steel and zinc industries have worked together for many years to perfect galvanized coatings capable of protecting steel from corrosion, regardless of the environment or application. From automobile body panels and underbody parts, appliances, gas pumps, mailboxes, street furniture, vineyard wire and buildings, the life-extension and finishing options made possible by modern zinc coatings are staggering.
But steel is not the only material to benefit from the unique properties of zinc. When the US National Aeronautics and Space Administration (NASA) scientists needed a coating that could withstand the extreme
—————————————————————————————————————-
Rob Putnam is Communications Manager at the International Lead Zinc Research Organization (ILZRO) in the USA – rputnam@ilzro.org. Michael Martin is a consultant to International Zinc Association (IZA) – 106026.522@compuserve.com.
Zinc oxide is used in the manufacture of paints,
rubber products, cosmetics, pharmaceuticals,
floor coverings, plastics, printing inks, soap,
storage batteries, textiles, electrical equipment,
…
temperatures of space travel, they turned to zinc oxide. Researchers were able to develop a zinc-based coating capable of withstanding thermal cycling between 180° C and –180° C, and the bombardment of ultraviolet exposure equivalent to 19,000 sun hours. This zinc oxide coating is now routinely used to protect components of spacecraft, which are some of the most technically advanced and complex machines ever made.
The Cassini space probe left earth in 1997 on a seven-year voyage to Saturn. Upon arrival at Saturn, the probe will conduct a four-year mission with fifty orbits and numerous satellite encounters. Several parts of the Cassini probe, not least of which its four-meter diameter High Gain Antenna system, are protected by a thermal control paint based on zinc oxide. This zinc-containing thermal coating is of critical importance to the probe because extreme temperatures will be encountered as the probe approaches within 0.61 astronomical units (AU1) of the sun, during Venus flybys, and will be as far as 10 AU from the sun as it orbits Saturn. A number of coatings were evaluated for the project, but the zinc oxide formula best met Mission requirements, including the ability to withstand extreme temperatures and ultraviolet exposure.
Zinc Undersea
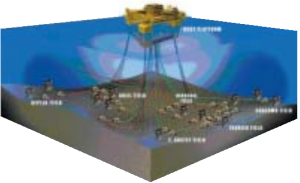
As offshore oil production moves to deeper water, conventional production platforms become too costly. The required mass of steel support structures grows disproportionately as the water depth increases. To overcome this problem, an alternative technique is to set wellheads on the seabed and control their operation remotely. A series of electrical and hydraulic lines are used to link the remote wellhead to a production platform located in shallow water closer to shore.
—————————————————————————————————————-
1 Astronomical Unit (AU) is the unit of distance used in the measurement of orbits and trajectories within the solar system. One AU is the average distance between the earth and the sun. Its value has been established as, roughly, 149,600,000 km (92,960,000 miles).
Zinc Entertains
Despite its many essential uses, sometimes zinc
is used purely for entertainment. Have you ever
wondered how things glow in the dark?All glow-in-
the-dark products contain phosphors.A phosphor is
a substance that radiates visible light after being
energized. To make a glow-in-the-dark object, you
want a phosphor that is energized by normal light
and that has a very long persistence. Zinc sulfide
has these properties and is used to create everything
from luminescent watch dials to glow-in-the-dark toys.
Zinc’s phosphorescent properties have also made it a
key ingredient in X-ray and TV screens, fluorescent
lights and light emitting diodes
Fireworks often make use of zinc dust to
create bright, shimmering sparks. The zinc
metal flakes heat up until they are incandescent
and shine brightly or, at a high enough temperature,
actually burn. A variety of chemicals can be added
to create the brilliant colors,
Until recently, these umbilical lines were made from high- pressure plastic hose, which proved unsatisfactory over long distances, or from metal alloys, which were cost prohibitive. Southwestern Pipe Inc., a U.S. company, in cooperation with Shell Oil and the International Lead Zinc Research Organization, developed a steel umbilical wrapped in a zinc cladding. The ductile zinc cladding is extruded to a thickness of 750 microns (standard zinc coatings range between 8 and 40 microns) and has a life of 30-40 years undersea. Today, the new zinc-protected umbilicals are commercialized under the name of SeaCAT. SeaCAT is now used on 75% of deepwater projects in the Gulf of Mexico, with over 11 million feet (3.4 million metres) of the tubing in service. SeaCAT is also playing a pivotal role in the largest sub-sea development in the world, the BP Amoco – Shell Na Kika project. This remarkable engineering feat will consist of five sub-sea developments, all tied back to a permanently-moored floating production facility. The wells are in water depths ranging from 5,800 to 7,000 feet (1,800 – 2,100 metres) and, when complete will require more than 1,000 miles (1,600 km) of zinc-clad tubing
BP Amoco – Shell Na Kika project
Zinc in Everyday Technology
Zinc castings are everywhere in today’s society, from light fixtures to faucets, from door handles to car parts. Radios, fax machines, computers and printers are but a few of our modern technologies that rely on diecast zinc parts.Recently developed casting techniques are fuelling the race for miniaturization of ‘next generation’ convenience appliances such as cell phones and laptop computers. As more technology is packed into tighter spaces, the need to remove heat from sensitive electronic components becomes critically important. Researchers have turned to zinc alloys to cast the miniature heat sinks needed to cool these appliances. Zinc oxide varistors are widely used in electrical systems for circuit protection. Commonly known as surge arresters, their primary function is to allow short-term circuit overloads to pass to ground. Most notably, varistors are used in high voltage power transmission lines as protection against lightning strikes and temporary power surges. They are also used in low voltage applications to protect sensitive components such as transistors and integrated circuits. Zinc plays a major role in the manufacture of the varistor. The internal component of the device is constructed almost entirely of zinc oxide. The outer shell or casing is usually a ceramic material or molded rubber.
To manufacture the internal zinc oxide block, zinc oxide is mixed with other metal oxides and a binder. This powder mix is subjected to high pressure and formed into a disc, which is placed in a kiln and baked for several hours. The zinc oxide particles fuse together to form a solid block. After a conductive coating is applied to the disc, multiple discs are stacked together and sealed in a shell. The size of the varistor varies in accordance with the voltage of the circuit to be protected. Zinc oxide in this ceramic form exhibits a unique electrical property – at design voltages, the block acts as a resistor, and at surge conditions, it acts as a conductor. Zinc is also a key ingredient in a new design of high-energy batteries. Researchers have already developed and commercialized a zinc-air battery capable of powering cell phones, hearing aids and laptop computers many hours longer than previously possible with other battery chemistries. Work is now ongoing to scale this and other zinc technology to a size suitable for powering automobiles and even homes. For example, the practice of truck engine idling, to heat or cool sleeper cabs when the truck is parked, may soon be replaced by zinc air fuel cells that provide quiet and clean auxiliary power for truck air-conditioning. America’s truckers currently spend as much as $4,000 per year per truck by idling engines to run auxiliary devices.Other uses for the ultra-clean and quiet zinc air fuel cell include forklifts and lawnmowers, scooters and delivery vehicles, back-up generators for the telecommunications industry and auxiliary power for boats, trucks and recreational vehicles, golf carts and industrial cleaning vehicles.
Zinc for Excellence
Each year, the U.S. National Academy of Recording
Arts & Sciences honors achievement in recorded music
with thepresentation of the Grammy® Award.The
Grammy is a peer honor, awarded annually, by and
to artists and technical professionalsfor artistic and
technical excellence.Befitting the recipient’s accomplishments,
the awards are hand-crafted from zinc alloysby an artisan
in Colorado.
exhibits a unique electrical property – at design voltages, the block acts as a resistor, and at surge conditions, it acts as a conductor.Zinc is also a key ingredient in a new design of high-energy batteries. Researchers have already developed and commercialized a zinc-air battery capable of powering cell phones, hearing aids and laptop computers many hours longer than previously possible with other battery chemistries. Work is now ongoing to scale this and other zinc technology to a size suitable for powering automobiles and even homes. For example, the practice of truck engine idling, to heat or cool sleeper cabs when the truck is parked, may soon be replaced by zinc air fuel cells that provide quiet and clean auxiliary power for truck air-conditioning. America’s truckers currently spend as much as $4,000 per year per truck by idling engines to run auxiliary devices.Other uses for the ultra-clean and quiet zinc air fuel cell include forklifts and lawnmowers, scooters and delivery vehicles, back-up generators for the telecommunications industry and auxiliary power for boats, trucks and recreational vehicles, golf carts and industrial cleaning vehicles.
Looking Ahead
Despite a long history, zinc is a uniquely modern metal. With its vast array of properties, zinc has few peers in terms of usefulness in the ever-expanding fields of science and technology. Its versatility has made it a basic medium for researchers as they probe new frontiers in physics, chemistry, biology and electrical engineering.With many thousands of patents and literally millions of products to its credit, zinc played a supporting role in many of the major inventions of the 20th Century. From transistors to lasers, satellites to circuit boards, photocopiers to fuel cells, zinc is truly among the most versatile and essential materials known to mankind.